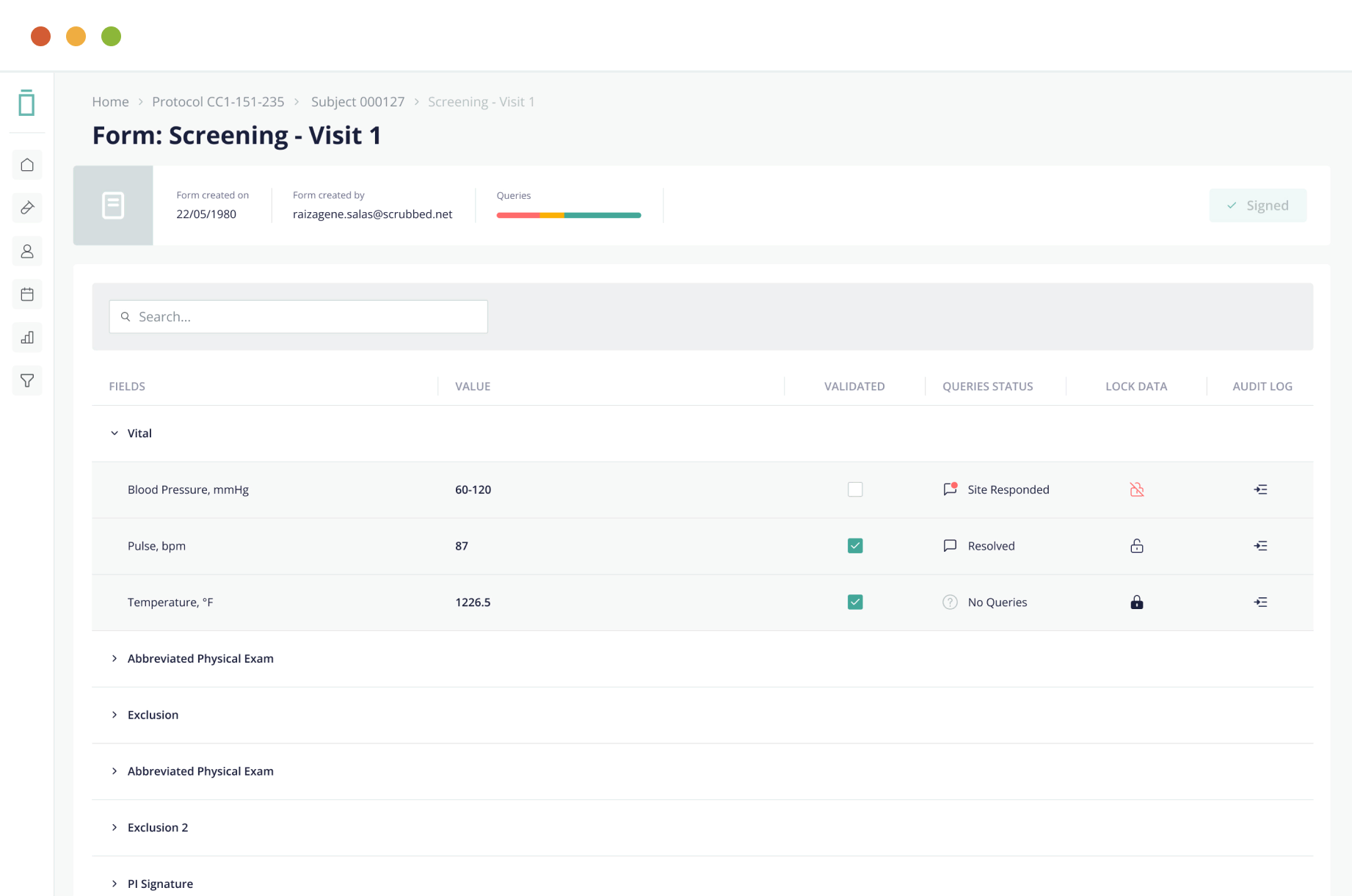
Intelligent data capture reduces protocol deviations for sites
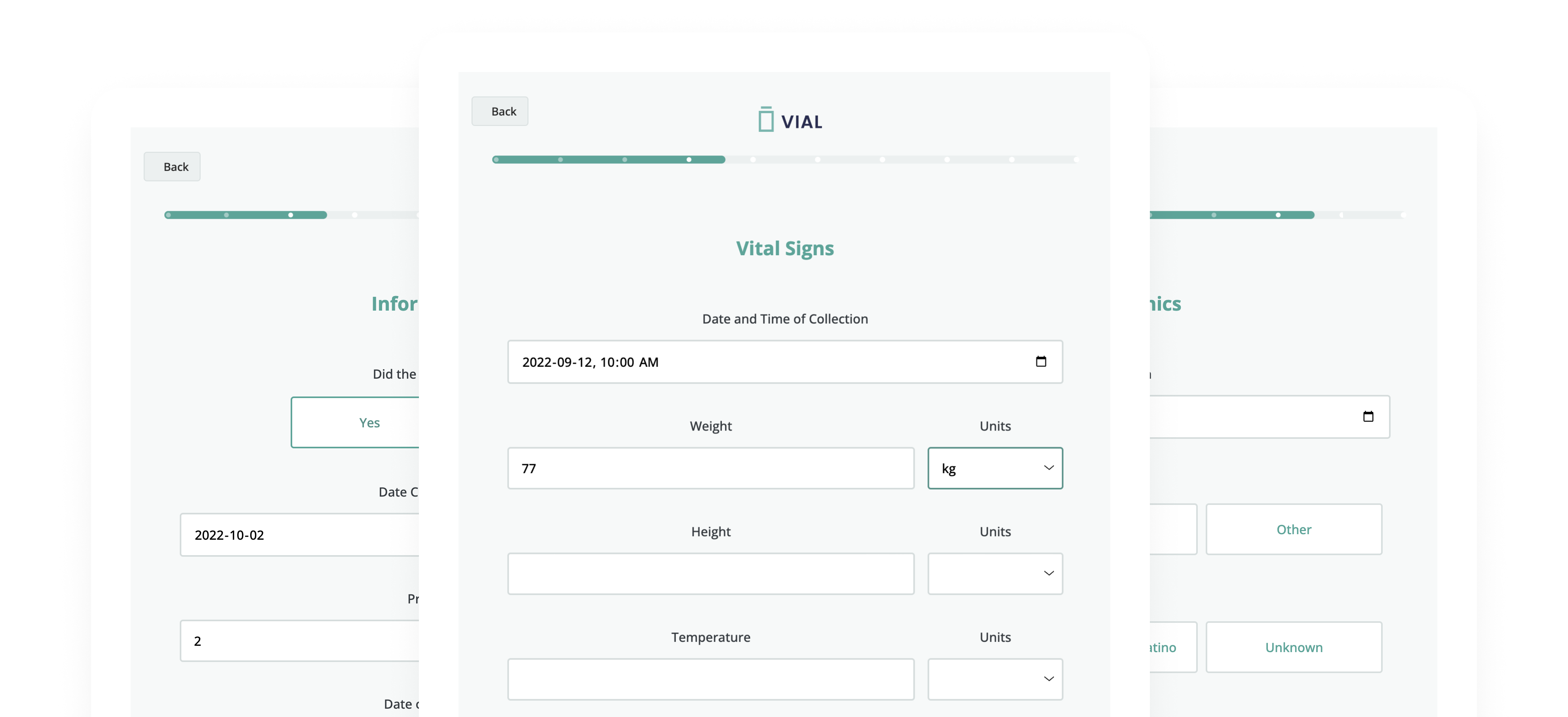
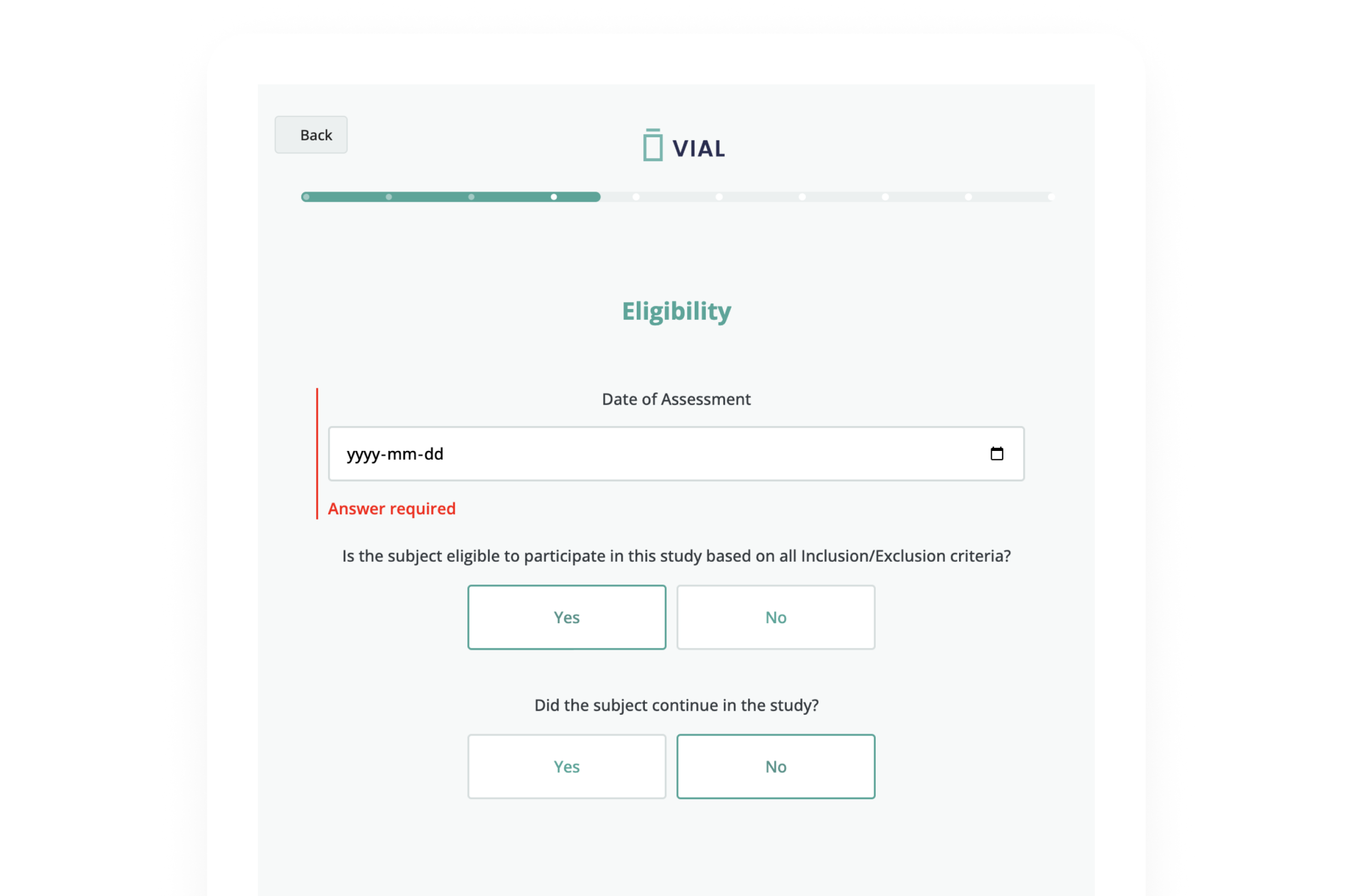
Vial eSource technology assures data quality through an intelligent, real-time data capture system. eSource data fields are built on CDISC standards using SDTM-controlled terminology for quality on-entry.