The Dermatology CRO for Biotech, Powered by Technology
Delivering faster, better, cheaper dermatology trial results for biotech sponsors.
Our dermatology CRO Executive Team
Our Dermatology CRO team's expertise spans across indications with prior experience at both large and small CROs
Our experienced CRO executive team works closely with our expert site operations team to continuously review execution strategies, closely monitor patient recruitment efforts, and mitigate key study risks for Dermatology clinical trials.

Orin Goldblum, MD
Powered By Our Connected Technology Platform
The Vial Technology Platform leverages connected systems and intuitive design to run global trials efficiently at scale
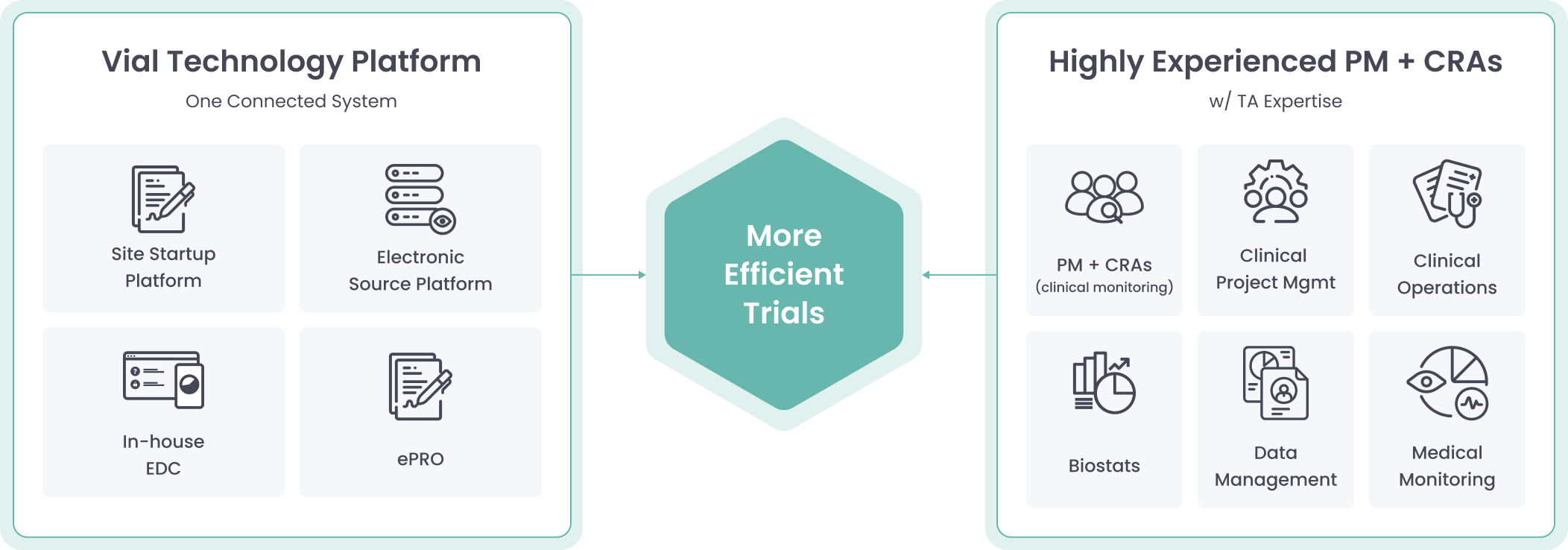
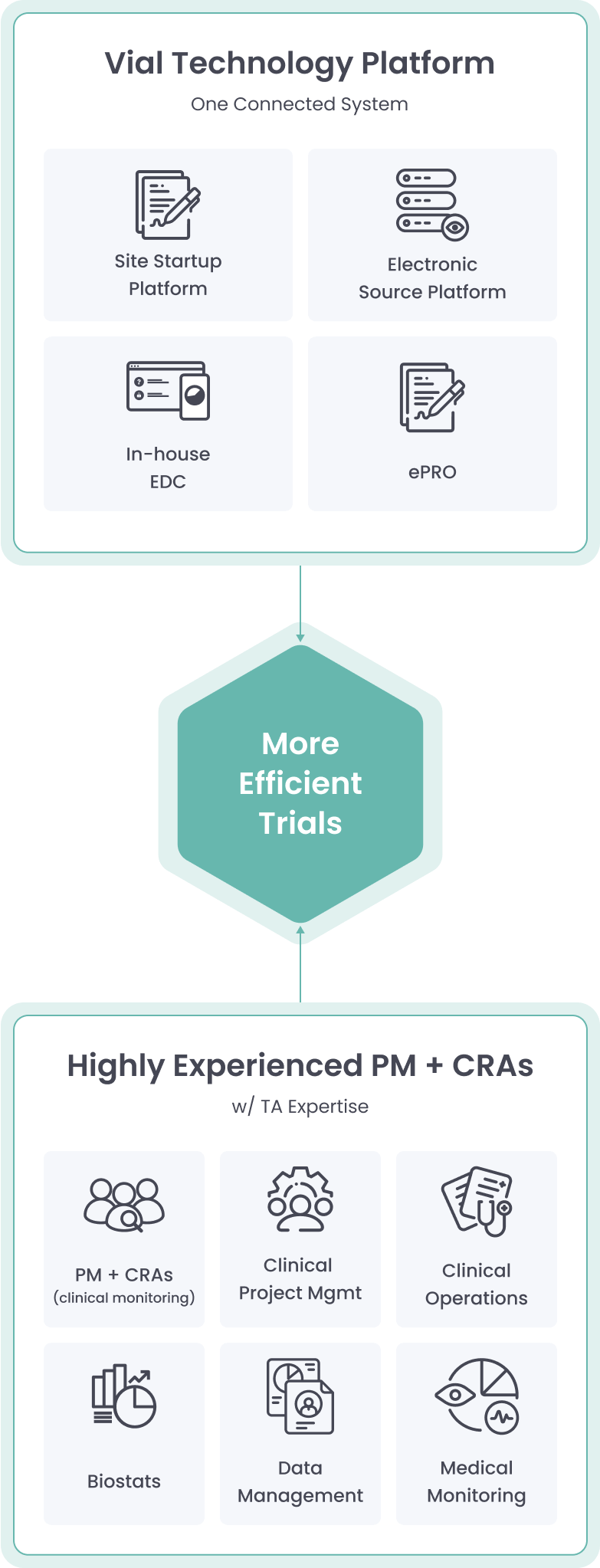
Vial’s modern technology platform brings clinical trials out of the paper stone age, allowing for streamlined processes inside one end-to-end system.
no-nonsense pricing
Vial offers sponsors fixed-pricing agreements with no change orders — ever.
Flat-rate pricing
A fixed-fee pricing model guarantees a set budget for the entirety of a project, regardless of the time and expense that accrues.
Zero change orders
Thorough planning, an experienced team, and our next-gen technology platform allow us to proactively prepare for and avert common errors or delays.
Risk-share on commitments
We focus on delivering results that matter, and our risk share on commitments approach underscores our dedication to your trial's success.
Fast Start-up and Consolidated Site Activation
Vial’s Site Startup Platform enables sites to seamlessly onboard to our trials
The Result: Self-service onboarding eliminating administrative burden and increasing progress transparency.
Scientific Advisory Board
Experience across Dermatology
Vial CRO is supported by a team of Scientific Advisors who review and provide input on the strategy and direction of the CRO in order to build the infrastructure to advance research.
vial dedicated phase I unit
Vial’s Dermatology Phase 1 unit provides a unified full service solution to early clinical development
Phase 1 Units are critical to advancing care and validating treatments, which is why it is essential to have a dependable Phase 1 unit you can rely on.
Vial’s dedicated Phase 1 research coordinators have upwards of 25 years of experience and are IATA and CPR certified. All of our research coordinators complete annual GCP and NIH training, as well as routine in-house training. In addition to extensive training, they are familiar with multiple EDC and IRT/IWRS systems, making them a well-rounded team you can trust.


Battery Bio
With Battery Bio, Vial’s in-house drug discovery division, we are demonstrating a low cost, tech enabled approach to running clinical trials

Discover insights to help you run trials faster and more efficiently.
Advancing dermatology Care
Dermatological conditions can have a significant impact on patients' quality of life.
Clinical trials are critical for developing new treatments and advancing care for patients with dermatological conditions, which affect their physical appearance, causing discomfort and pain, and impacting mental health. Vial offers dermatology sponsors solutions that enable them to improve the efficiency of their clinical trials and accelerate the development of effective therapies. By improving patient outcomes and quality of life, Vial CRO's support contributes to advancing dermatology research and treatment options.


Connect with us.
Interested in receiving a proposal from Vial? Leave us a message and some of your contact info and we’ll be in touch with you shortly.