Next Generation Pharma Company
Next generation pharma company powered by computationally designed therapeutics and automated trials
AI Designed Therapeutics

Target Discovery
Vial’s integrated models analyze massive multi-omic and clinical datasets to identify high-value therapeutic targets with unprecedented speed and resolution.

Computational Molecule Design
Our platform generates, evaluates, and optimizes drug candidates through iterative AI-driven design loops, reducing timelines from months to days.

Rapid Experimental Validation
Vial’s automated wet-lab workflows and high-throughput assays validate AI-designed compounds quickly, enabling faster prioritization and clinical translation.
Vial’s approach to R&D brings therapeutics to life with unprecedented speed and scale
- AUTOMATED LABS
Automated Clinical Trials
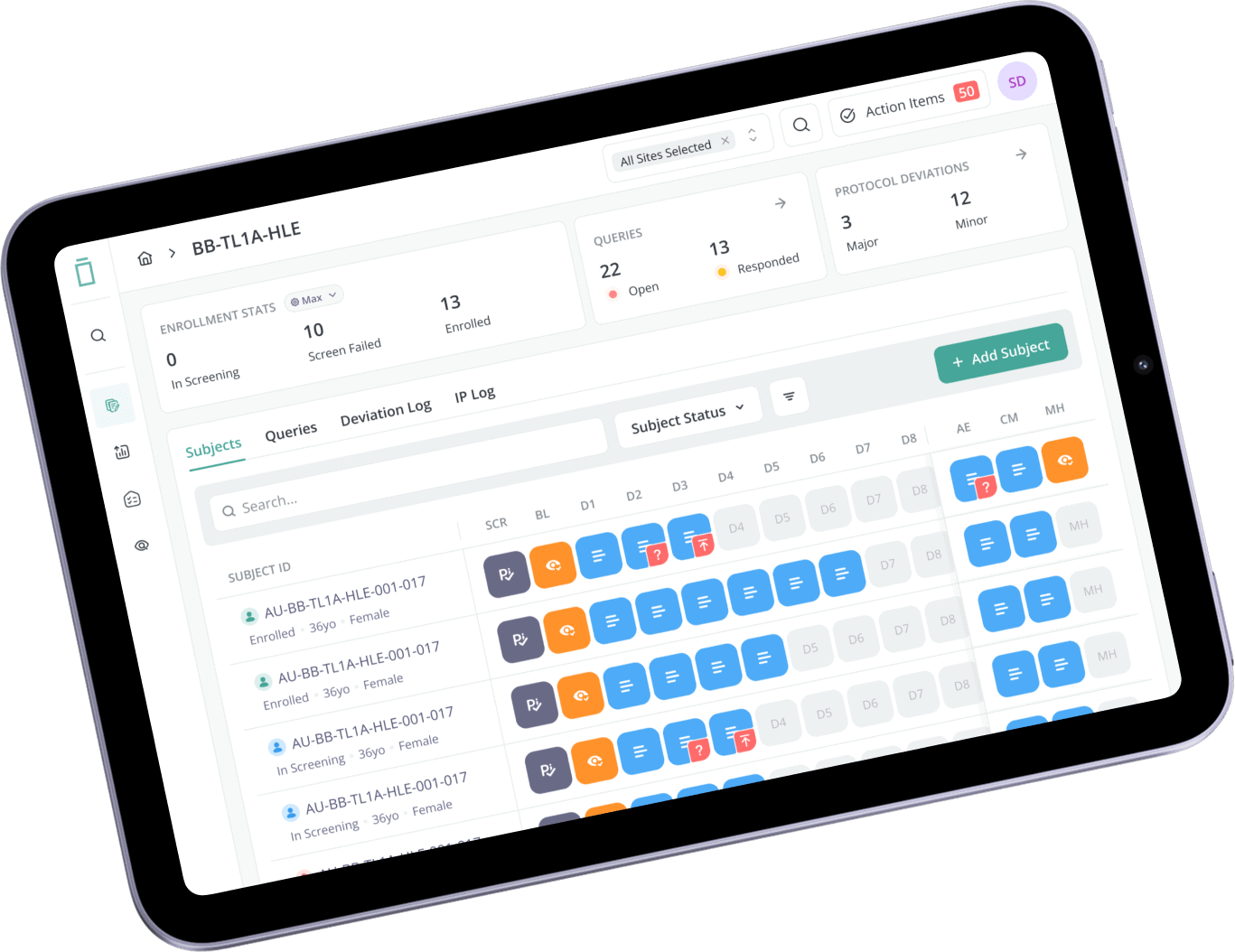
- MASSIVE SCALE
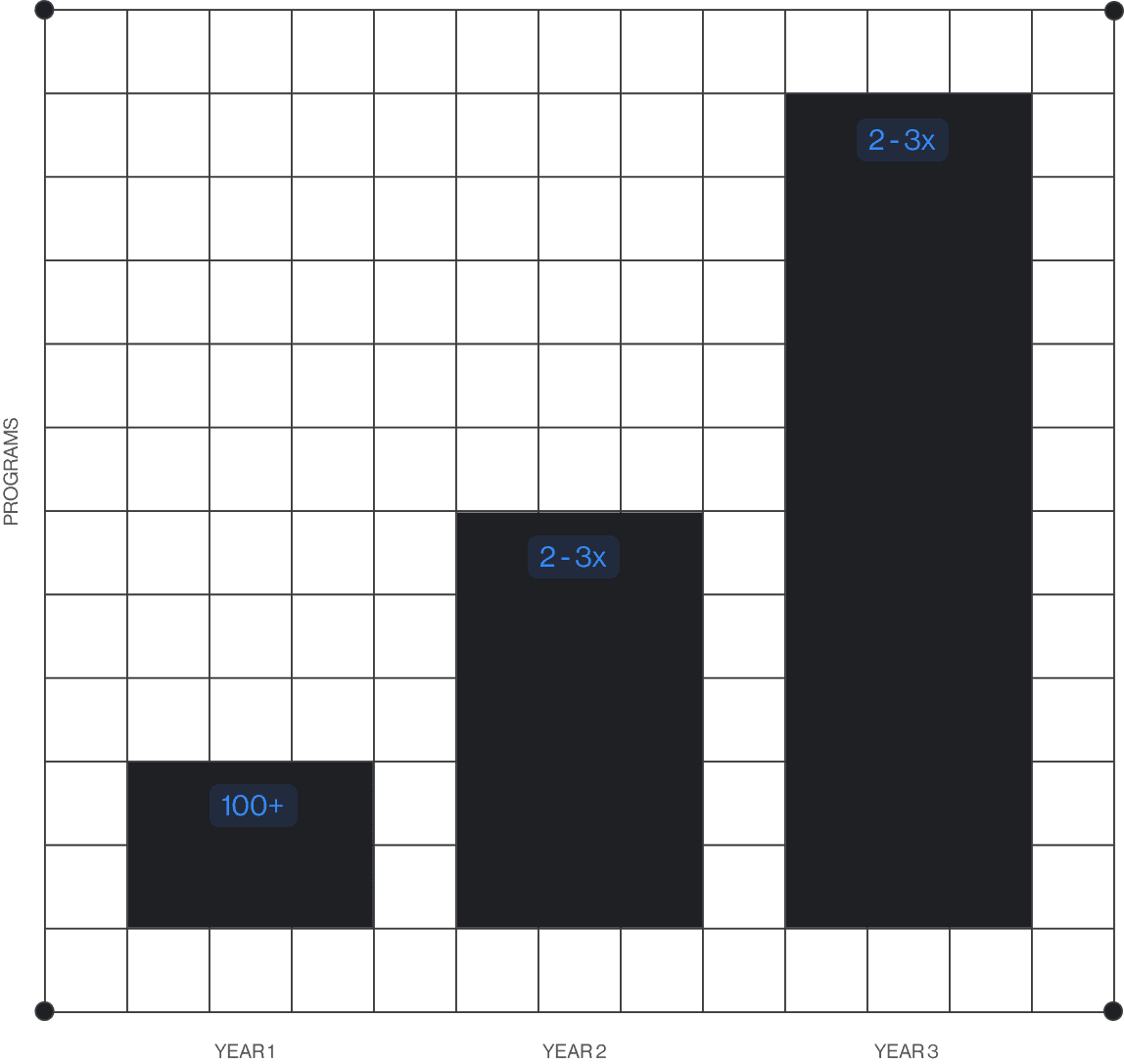
Clinical Trials at Massive Scale
We believe massively scalable trial execution will be one of the defining capabilities of the next decade. The speed, reliability, and systemization of Vial empowers us to operate complex portfolios in parallel, reduce operational overhead, and accelerate the development of new therapies.
Focus Areas
Contact
Get in touch!
"*" indicates required fields