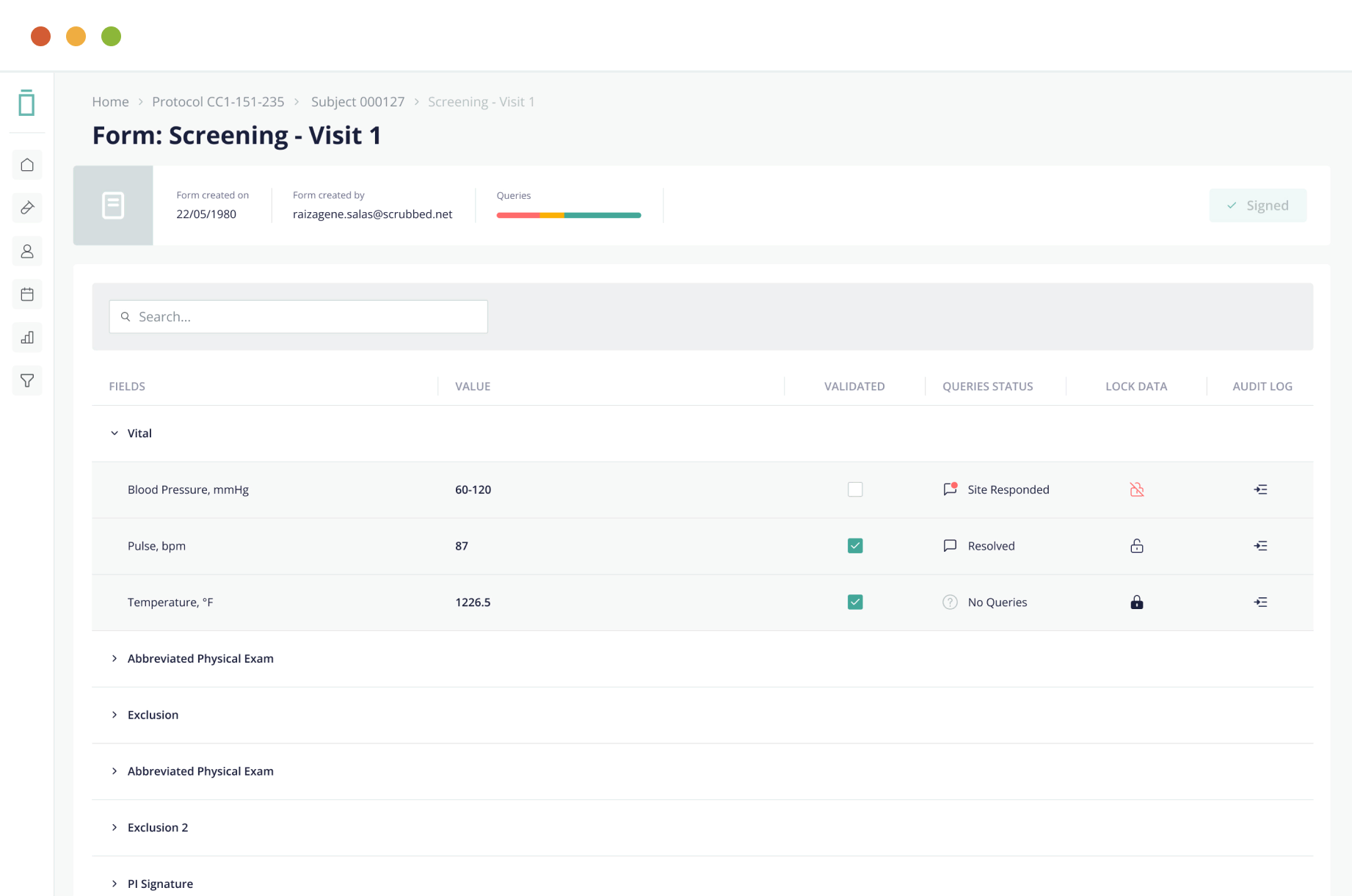
Data Capture Made Easy
Vial EDC offers an intuitive UI/UX design with a reliable and scalable cloud-based platform that enables simple, real-time eCRF data input and structuring for accelerated performance.
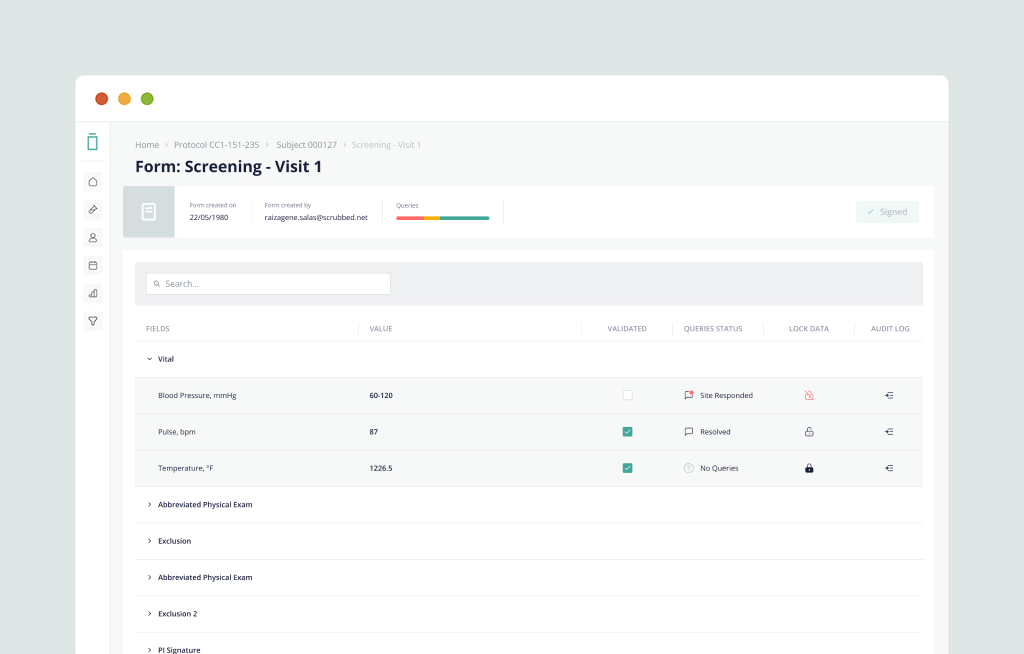
Delivering a consumer-grade experience and next-generation performance to eClinical software. Vial EDC is a modern, intuitive, and hyper-responsive EDC platform that sites love.








Vial EDC offers an intuitive UI/UX design with a reliable and scalable cloud-based platform that enables simple, real-time eCRF data input and structuring for accelerated performance.

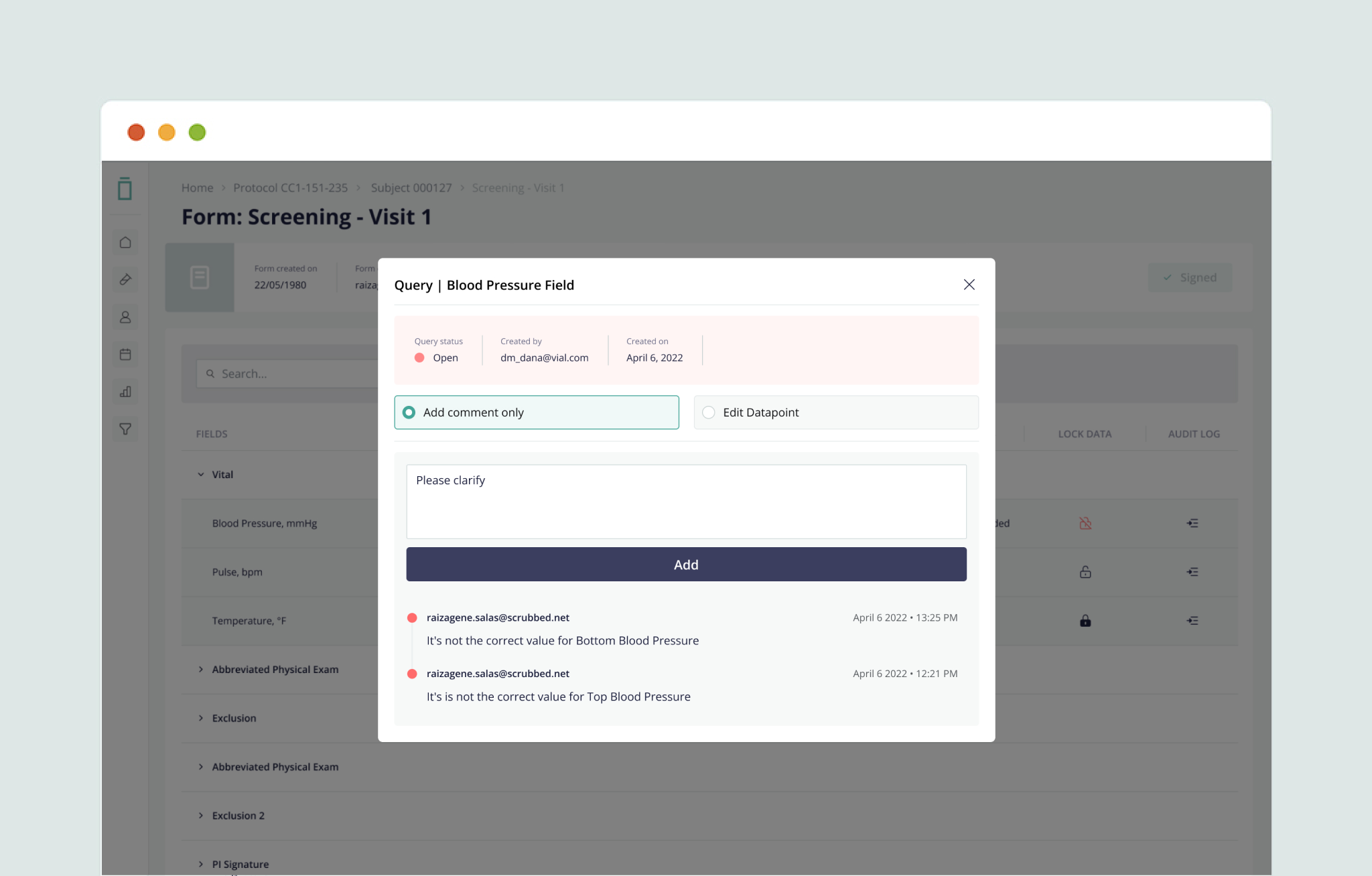
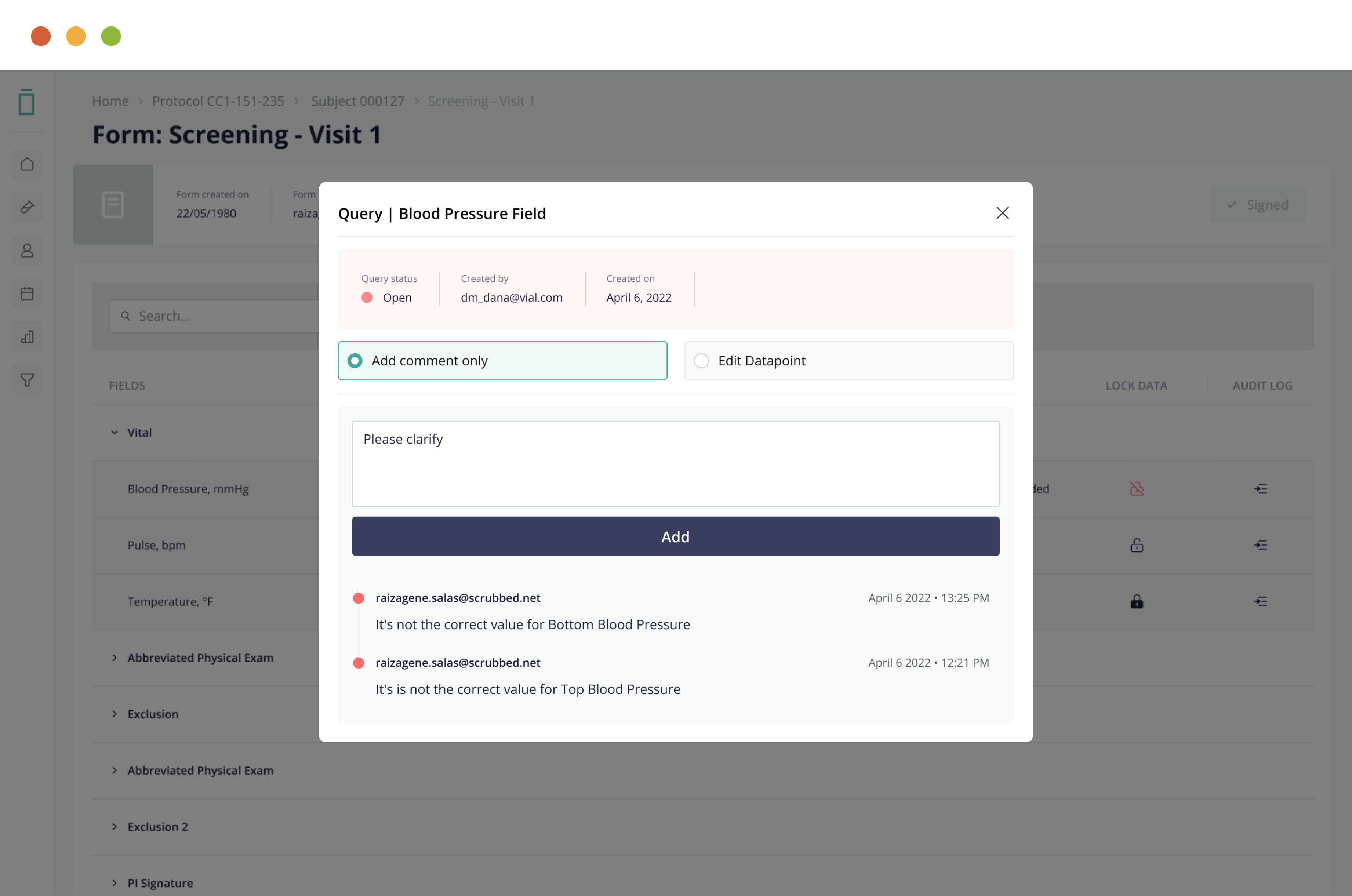
Streamline data cleaning and source data verification through full data query, audit, freeze, and lock features—ensuring data integrity.
Built for security and scale
The Vial Technology Platform is built on a cloud-based infrastructure with a robust authentication & permissions engine. Vial EDC is fully encrypted and compliant with the standards of 21 CFR Part 11, HIPAA, and GDPR.
Fast, Quality-Driven Build Process
The Vial Technology Platform can run global trials across multiple therapeutic areas efficiently at scale. Our highly-customizable source creation with multi-language support, coupled with lightning-fast build times, delivers best-in-class clinical trial results for sponsors.
The Vial Technology Platform can run global trials across multiple therapeutic areas efficiently at scale. Our highly-customizable source creation with multi-language support, coupled with lightning-fast build times, delivers best-in-class clinical trial results for sponsors.
Electronic Data Capture (EDC) is an electronic system used to record and maintain subject data in clinical trials. EDCs often contain electronic case report forms (eCRF). Common features of EDC systems include a data entry system, validation process, and reporting tools for data analysis.
1. Fully-Flexible and Customizable eSource
2. eSource Data Validation & Conditional Logic On-Entry
3. Fast Database & eCRF Builds (~8 weeks)
4. Real-Time Data Input & Storage to EDC
5. Full Query Management Capabilities
6. Data Export & Report Features
7. Flexible and Fast Data Integrations: IRT & ePRO
8. Encrypted and Compliant: 21 CFR Part 11, HIPAA, GDPR
9. Web-Based for Zero Installation & Secure Access
10. Cloud-Native Infrastructure: Global-Ready, Built-In Data Protection
11. Personalized Software Support & Training
Leave us a message and some of your contact info and we’ll be in touch with you shortly.