The Ophthalmology CRO for Biotech, Powered by Technology
Delivering faster, better, cheaper ophthalmology trial results for biotech sponsors.
Our Ophthalmology CRO Executive Team
Our Ophthalmology CRO team's expertise spans across indications with prior experience at both large and small CROs
Our experienced CRO executive team works closely with our expert site operations team to continuously review execution strategies, closely monitor patient recruitment efforts, and mitigate key study risks for Ophthalmology clinical trials.

Amy Del Medico

Dr. Anton Kolomeyer

Dr. Charles Tressler
Powered By Our Connected Technology Platform
The Vial Technology Platform leverages connected systems and intuitive design to run global trials efficiently at scale
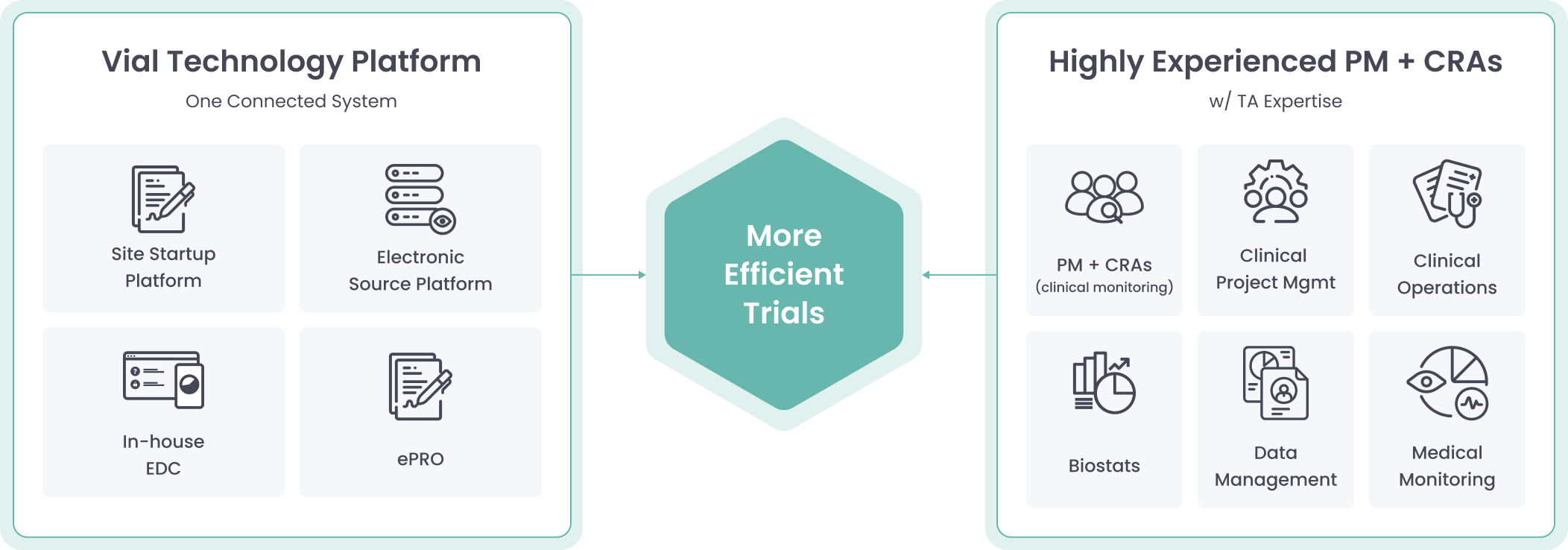
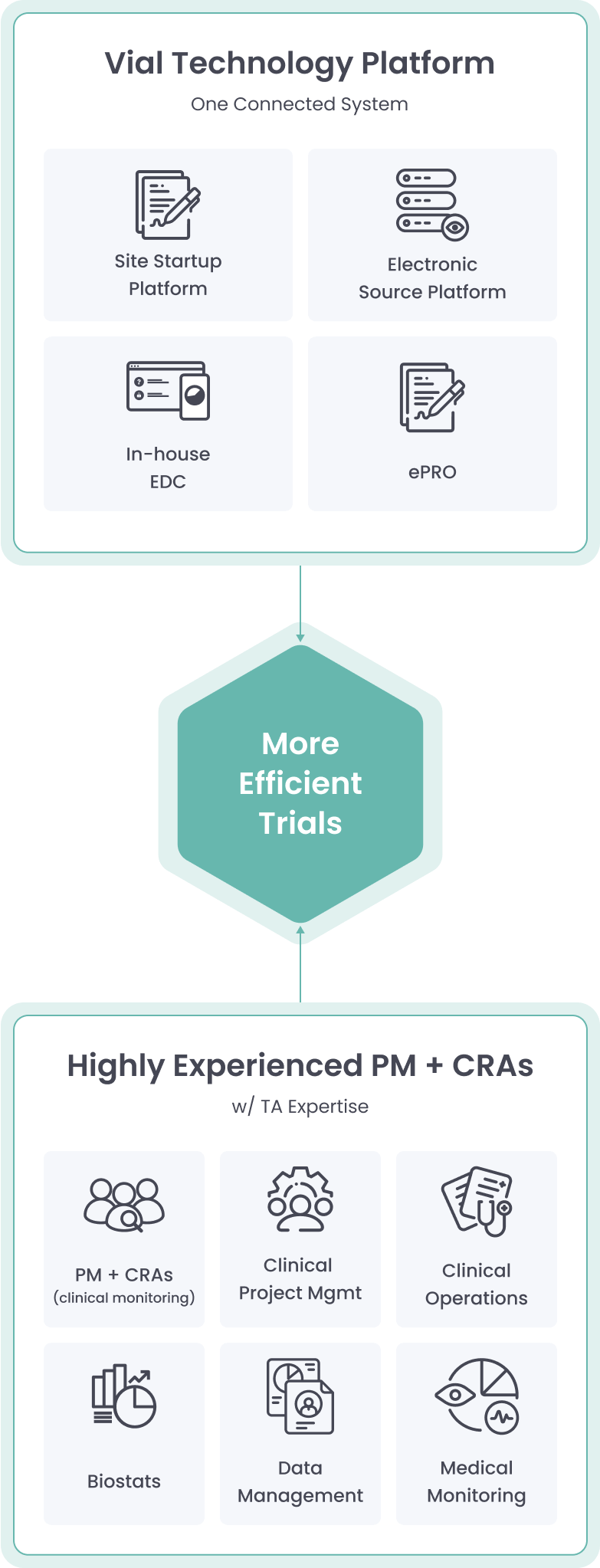
Vial’s modern technology platform brings clinical trials out of the paper stone age, allowing for streamlined processes inside one end-to-end system.
no-nonsense pricing
Vial offers sponsors fixed-pricing agreements with no change orders — ever.
Flat-rate pricing
A fixed-fee pricing model guarantees a set budget for the entirety of a project, regardless of the time and expense that accrues.
Zero change orders
Thorough planning, an experienced team, and our next-gen technology platform allow us to proactively prepare for and avert common errors or delays.
Risk-share on commitments
We focus on delivering results that matter, and our risk share on commitments approach underscores our dedication to your trial's success.
Fast Start-up and Consolidated Site Activation
Vial’s Site Startup Platform enables sites to seamlessly onboard to our trials
The Result: Self-service onboarding eliminating administrative burden and increasing progress transparency.
Scientific Advisory Board
Experience across Ophthalmology
The Vial CRO is supported by a team of ophthalmic scientific advisors who provide expert input to advance ophthalmology research.
Battery Bio
With Battery Bio, Vial’s in-house drug discovery division, we are demonstrating a low cost, tech enabled approach to running clinical trials

Discover insights to help you run trials faster and more efficiently.
why the vial ophthalmology cro?
Streamlined trial startup, more predictable patient recruitment, and higher quality data.
Vial understands the nuances in running Ophthalmology clinical trials and have built a team of in-house Ophthalmologists with decades of combined experience across anterior and posterior segments.
Our advisors’ experience in every Ophthalmology clinical trial indication, combined with a preferred site network of top-tier ophthalmologists, allow for a fully integrated Ophthalmology CRO that offers faster trial startup, superior patient recruitment, and better quality data.

Connect with us.
Interested in receiving a proposal from Vial? Leave us a message and some of your contact info and we’ll be in touch with you shortly.