Reimagining Oncology Dermatology Ophthalmology GastroenterologyCNS Biotech Clinical Trials
Reimagining Clinical Trials
We deliver on this vision by delivering faster, more efficient trials
Powered by our experienced ClinOps and technology leaders, we have become a leading global, full service CRO.
Oncology
Dermatology
Ophthalmology
Gastroenterology
CNS
Cardiology
The Vial technology platform leverages connected systems and intuitive design to run global trials efficiently at scale
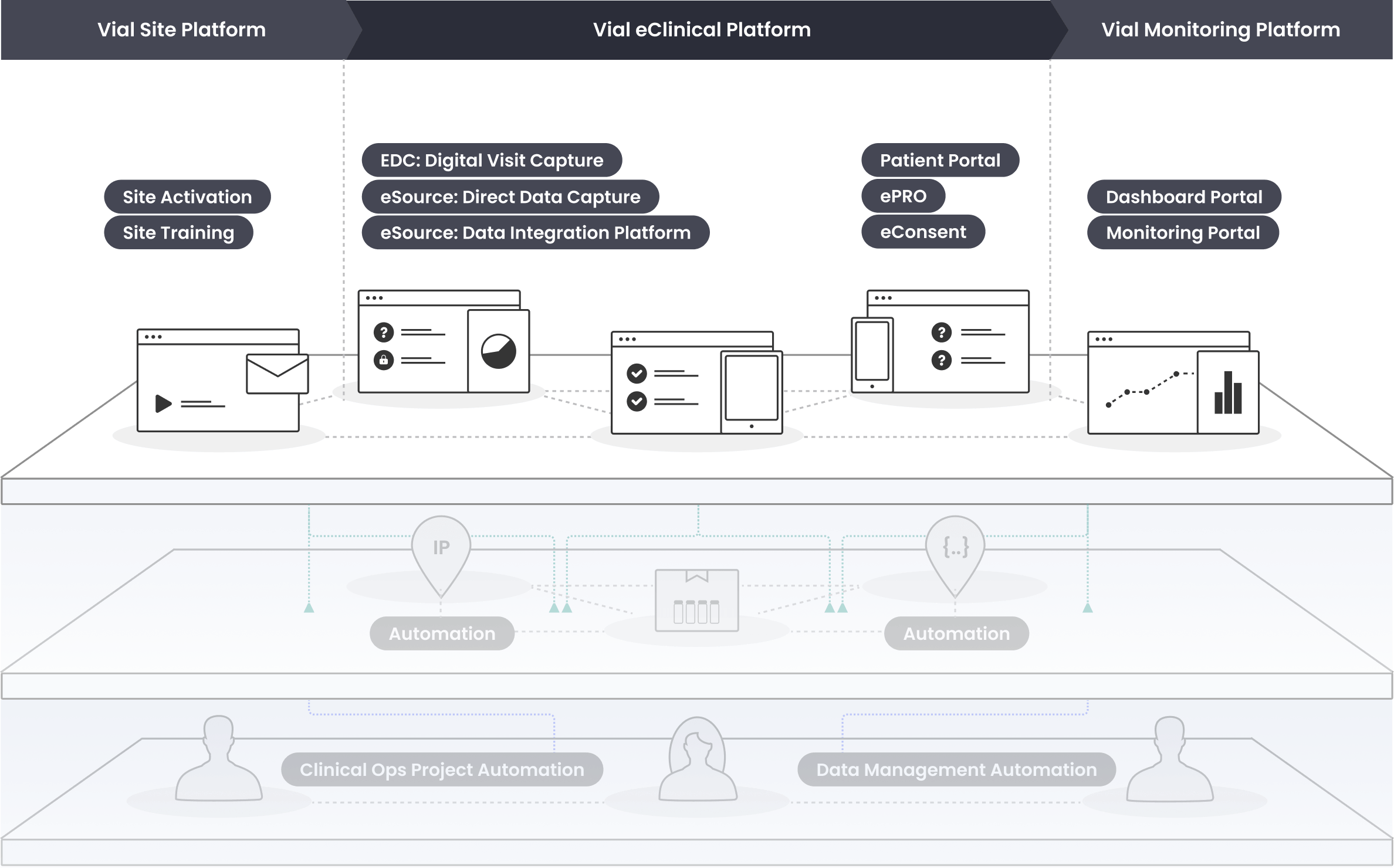
Vial’s modern technology platform brings clinical trials out of the paper stone age, allowing for streamlined processes inside one end-to-end system.
Battery Bio
With Battery Bio, Vial’s in-house drug discovery division, we are demonstrating a low cost, tech enabled approach to running clinical trials

Vial has a leading team of experts in various therapeutic areas
Our clinical operations expertise paired with Vial’s technology platform delivers dramatically faster and more efficient trials for sponsors.

Wendy Pinson

Rich McCormick

Breanna Wallace

Sydney Cobb

Carrie Williams

Laura Whitlock

Paschal Okeke

Gold Emeh

Anita Ebeye

Orin Goldblum

Johnbosco Umejiego

Anton Kolomeyer

Dennis Fabia

Charles Tressler

Anna Spagnoli

Etunim Mike

Kelly Croom

Enejo Oruma
We’re building a technology platform and products we believe in.
our Vision
Our vision is to empower scientists to cure all human disease. We execute on this vision by delivering faster, more efficient trials, powered by our experienced ClinOps and technology platform.
our mission
Vial's culture is one of highly autonomous, deeply focused experts collaborating on immensely challenging problems together. We strive to be adaptive and work cohesively as a team to solve problems at every turn.

Discover insights to help you run trials faster and more efficiently.
Connect with us.
Interested in receiving a proposal from Vial? Leave us a message and some of your contact info and we’ll be in touch with you shortly.