The Gastroenterology CRO for Biotech, Powered by Technology
Delivering faster, better, cheaper gastroenterology trial results for biotech sponsors
Powered By Our Connected Technology Platform
The Vial Technology Platform leverages connected systems and intuitive design to run global trials efficiently at scale
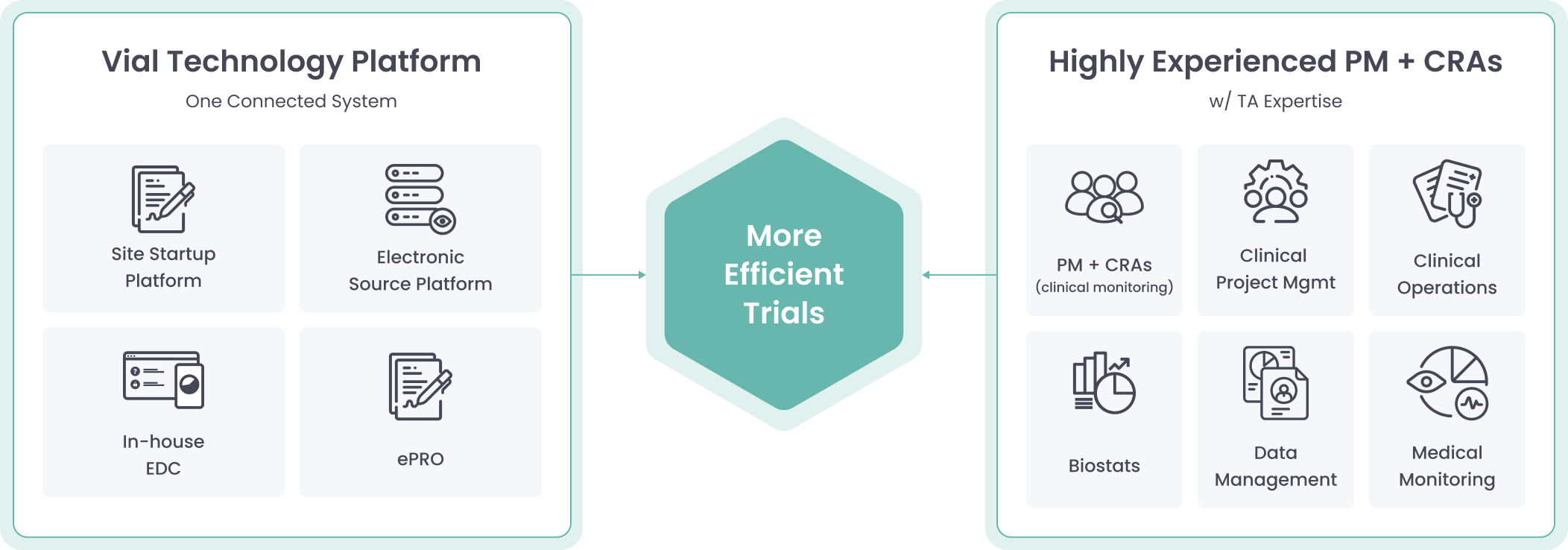
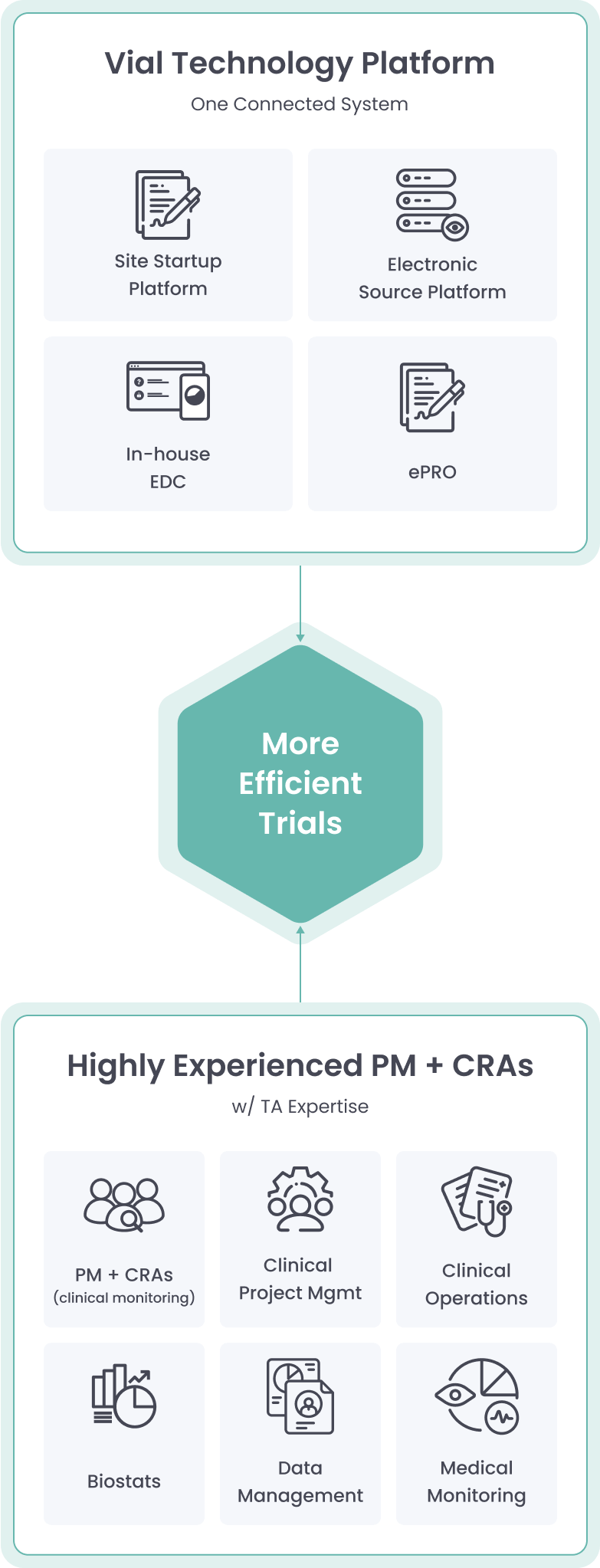
Vial’s modern technology platform brings clinical trials out of the paper stone age, allowing for streamlined processes inside one end-to-end system.
no-nonsense pricing
Vial offers sponsors fixed-pricing agreements with no change orders — ever.
Flat-rate pricing
A fixed-fee pricing model guarantees a set budget for the entirety of a project, regardless of the time and expense that accrues.
Zero change orders
Thorough planning, an experienced team, and our next-gen technology platform allow us to proactively prepare for and avert common errors or delays.
Risk-share on commitments
We focus on delivering results that matter, and our risk share on commitments approach underscores our dedication to your trial's success.
Fast Start-up and Consolidated Site Activation
Vial’s Site Startup Platform enables sites to seamlessly onboard to our trials
The Result: Self-service onboarding eliminating administrative burden and increasing progress transparency.
Battery Bio
With Battery Bio, Vial’s in-house drug discovery division, we are demonstrating a low cost, tech enabled approach to running clinical trials

Discover insights to help you run trials faster and more efficiently.
Advancing Gastroenterology Care
Gastrointestinal (GI) diseases are a significant health burden globally, leading to increased healthcare costs and decreased productivity.
Clinical trials are essential for developing new treatments and advancing care for patients with GI conditions. Vial offers GI sponsors solutions to support their research, including study design optimization, innovative patient recruitment strategies, and advanced data analytics techniques. With Vial's support, sponsors of GI clinical trials can bring new treatments to market faster and more efficiently, improving patient outcomes and quality of life.


Connect with us.
Interested in receiving a proposal from Vial? Leave us a message and some of your contact info and we’ll be in touch with you shortly.