TL1A
- Preclinical data support the potential for best-in-class dosing interval for patient convenience and efficacy among anti-TL1A therapies
- A Phase 1 open-label study in Australia will evaluate safety, pharmacokinetics, and pharmacodynamic responses in healthy volunteers
- Interim subcutaneous safety and pharmacokinetic data from healthy volunteers expected in H1 2026
Key Data
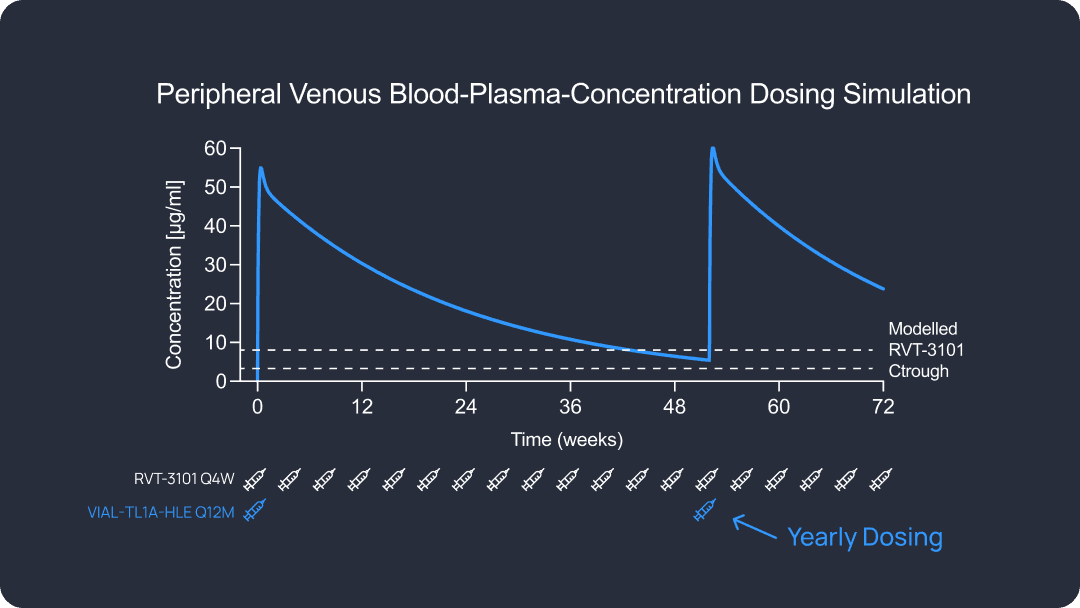
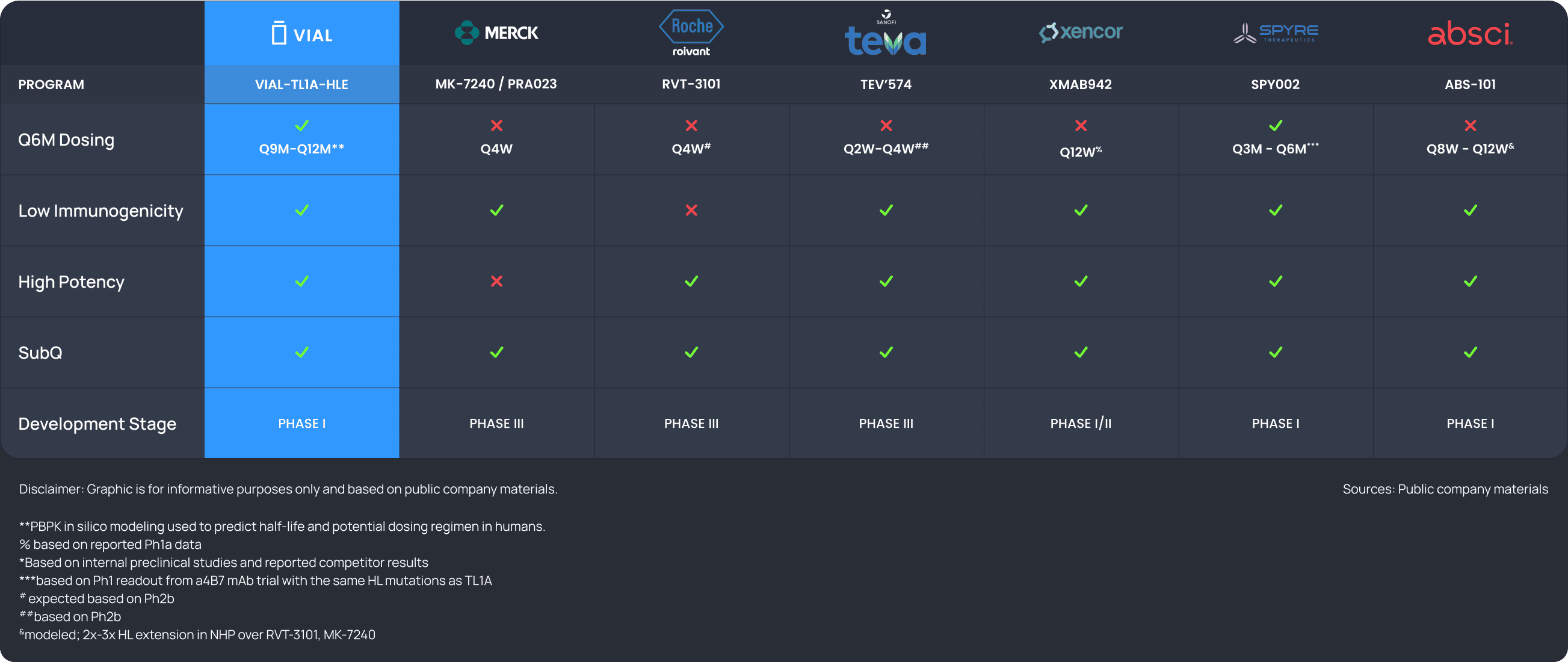
Preclinical data indicate a best-in-class dosing interval for patient convenience and efficacy compared to the first and second-generation anti-TL1A candidates.
Equal or more potent than competitors based on in vitro assays. Potency in line with first line anti-TL1A mAbs demonstrated by neutralization of apoptosis in TF-1 cells.
Low immunogenicity risk predicted by pre-clinical models.
Model dosing frequency would reduce patient burden. PBPK modeling suggesting industry leading predicted half-life.
Differentiated Product Profile
VIAL-TL1A-HLE is potentially a best-in-class anti-TL1A mAb with an extended half-life to support Q9-12M dosing, powered by Vial’s HLE platform. The program is also applicable to MASH, Atopic Dermatitis, SSc-ILD, Rheumatoid Arthritis, Hidradenitis Suppurativa, among others.
Updates
So 1st of all, TL1A Is one of the hot targets at the moment for both Crohn's and Uc. And one of the major challenges that we have in the clinic is to have drugs that need frequent administration and patients like to have drugs that are very effective and that are super safe, and that are easy to administer.
So instead of every 2 weeks, every month, having an injection every 6 months, or once a year, with a very safe mechanism of action. I mean, this is the ideal thing to have for a patient perspective and also for a doctor.
A long half-life reduces the burden on the patient hopefully provides a very steady state concentration of drug and binding of the target over the extended half-life, which in theory should be very good at preventing episodic flares.
I mean, IBD is a lifelong condition that requires long-term management. Many patients struggle with treatment, fatigue, adherence, challenges, and disruptions in daily life. So an extended half-life TL1A therapeutic, especially one with the potential for once yearly dosing, could significantly improve the quality of life, of reducing injection burden, minimizing clinic visits and maintaining consistent disease control.