Now recruiting sites globally
Now selecting sites for our Phase II IBD trial!
Phase II Study of TL1A HLE
Eligible Countries
Pending
At a glance
Primary objective
Evaluation of safety and efficacy based on endoscopic improvement (Mayo endoscopic subscore ≤1, no friability) at Week 12
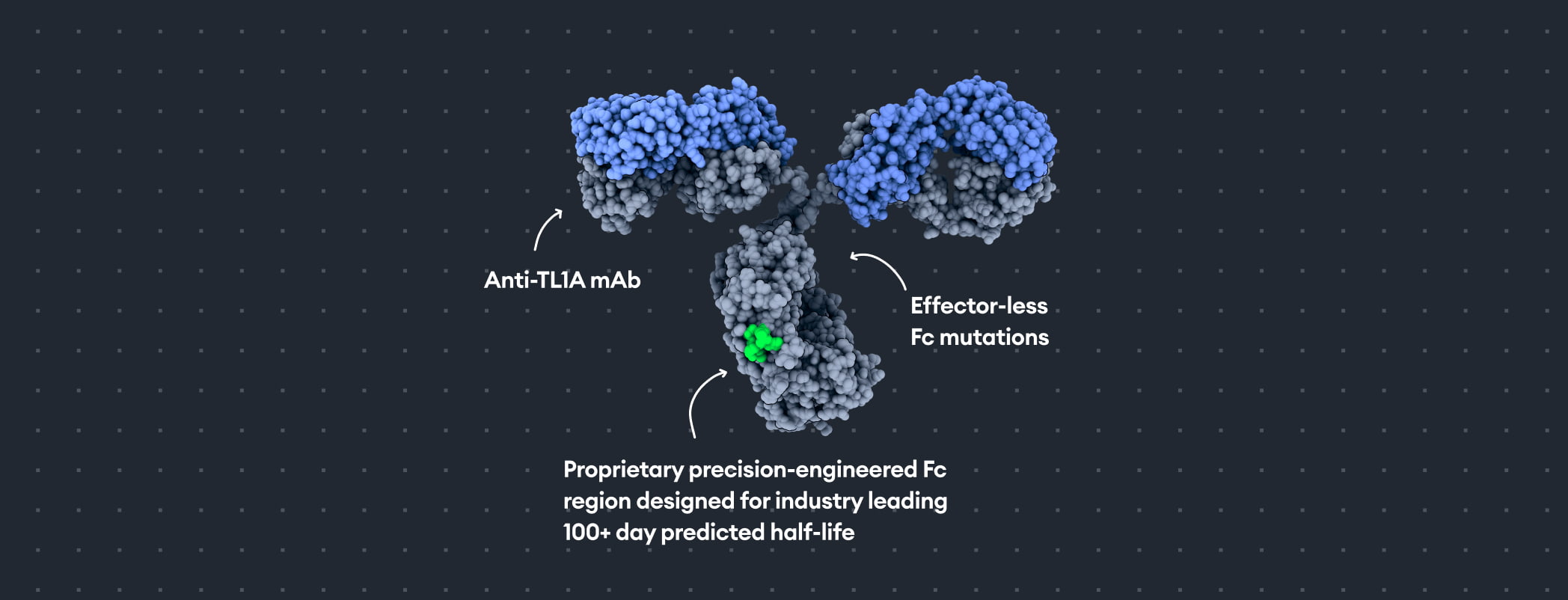
Mechanism of action
A half-life extended MAb that targets TL1A - DR3 and TL1A - DcR3 signaling
How many participants are being enrolled?
76
How long is participation in the clinical trial?
51 weeks
When is the clinical trial being conducted?
Q4 2025 / Q1 2026
Are placebos part of the clinical trial?
No, this is a single-arm trial where all participants receive the investigational product
About this clinical trial
The main aim of this study is to evaluate safety and efficacy of Vial’s half-life extended anti-TL1A antibody and how well it is tolerated in participants with moderate-to-severe ulcerative colitis. All participants will be treated with the investigational product if the participants meet the study requirements. Participants will be in the study for up to 51 weeks, 12 weeks for an induction period, and 39 weeks for clinical follow-ups. This timeline does not include pre-screening and screening period, which may require up to an additional 6 weeks prior to the start of the study period. During the study, participants will visit their study clinic multiple times.
Site Requirements
GI Trial Experience
Efficient Site Team
Remote Monitoring
Enrollment Requirements
Indication
Moderate-to-Severe Ulcerative Colitis
Age
18+ years
Sexes
All
Healthy Volunteers?
No
Biologically naive?
Yes
Biologically experienced?
Yes
Key Inclusion & Exclusion Criteria
- Must be 18 years of age or older
- Must have a body weight of at least 50kg and a BMI between 18 - 32 kg/m2 (inclusive)
- Must be diagnosed with ulcerative colitis for at least 3 months
- Must have moderate-to-severe ulcerative colitis as defined by a modified aggregate UC severity score of at least 4, a endoscopic severity score of at least 2, and a rectal bleeding sub-score of at least 1
- Cannot have an active, serious infection requiring IV antibiotics within 4 weeks prior to enrollment
- Cannot have taken investigational drugs or devices within 30 days of baseline efficacy assessments or 5 half-lives, whichever is longer
- Cannot have taken biologics within 12 weeks of baseline efficacy assessments or 5 half-lives, whichever is longer
Outcome measures
Primary outcomes
- % of participants who achieve endoscopic improvement at 12-weeks post dose
- Incidence of dose limiting or intolerable treatment related adverse events and serious adverse events
Secondary Outcomes
- % of participants who achieve clinical remission at 12-weeks pose dose
- % of participants who achieve clinical response at 12-weeks pose dose
- % of participants who achieve symptomatic remission at 12-weeks pose dose
Interested in joining this clinical trial?
Submit the form and our team will be in touch with the feasibility questionnaire and information on next steps.