IL-13 x TSLP
- DIO study in progress to validate that fat loss/lean mass ratios are in line with best-in-class clinical INHBE programs, with key readouts in August 2025.
- Entering IND-enabling studies in Q3 2025.
- Phase I initiating in Q4 2025.
Key Data
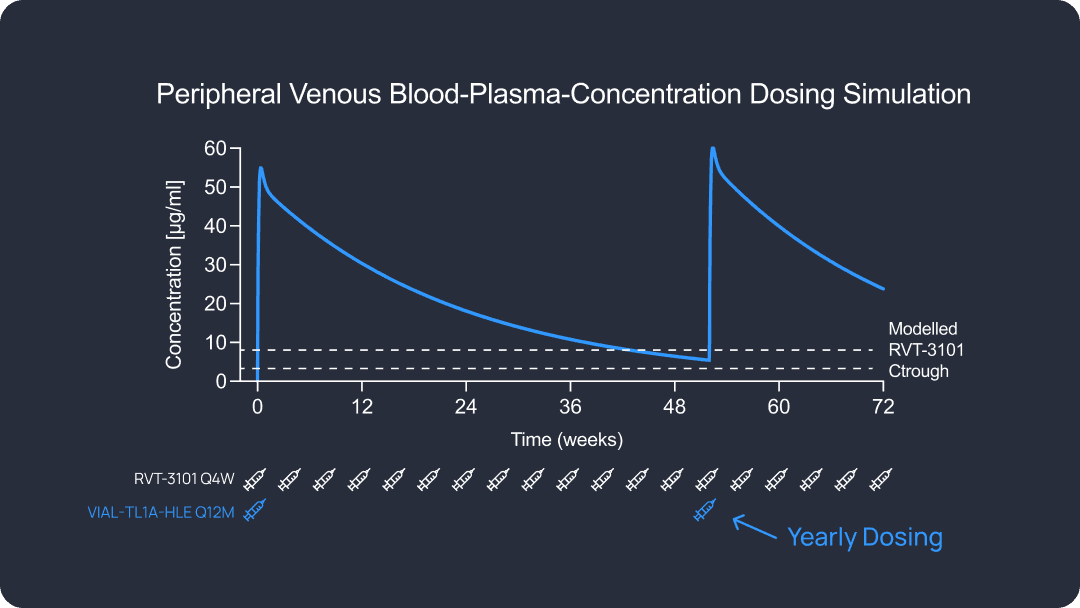
Preclinical data indicate a best-in-class dosing interval for patient convenience and efficacy compared to the first and second-generation anti-TL1A candidates.
Maximally inhibits CCL17 production as compared to marketed anti-IL13 or anti-TSLP mAbs alone.
Developability, a common issue with bi-specifics especially those that have half-life extension, has been de-risked through extensive molecule optimization and validated through preclinical testing.
Differentiated Product Profile
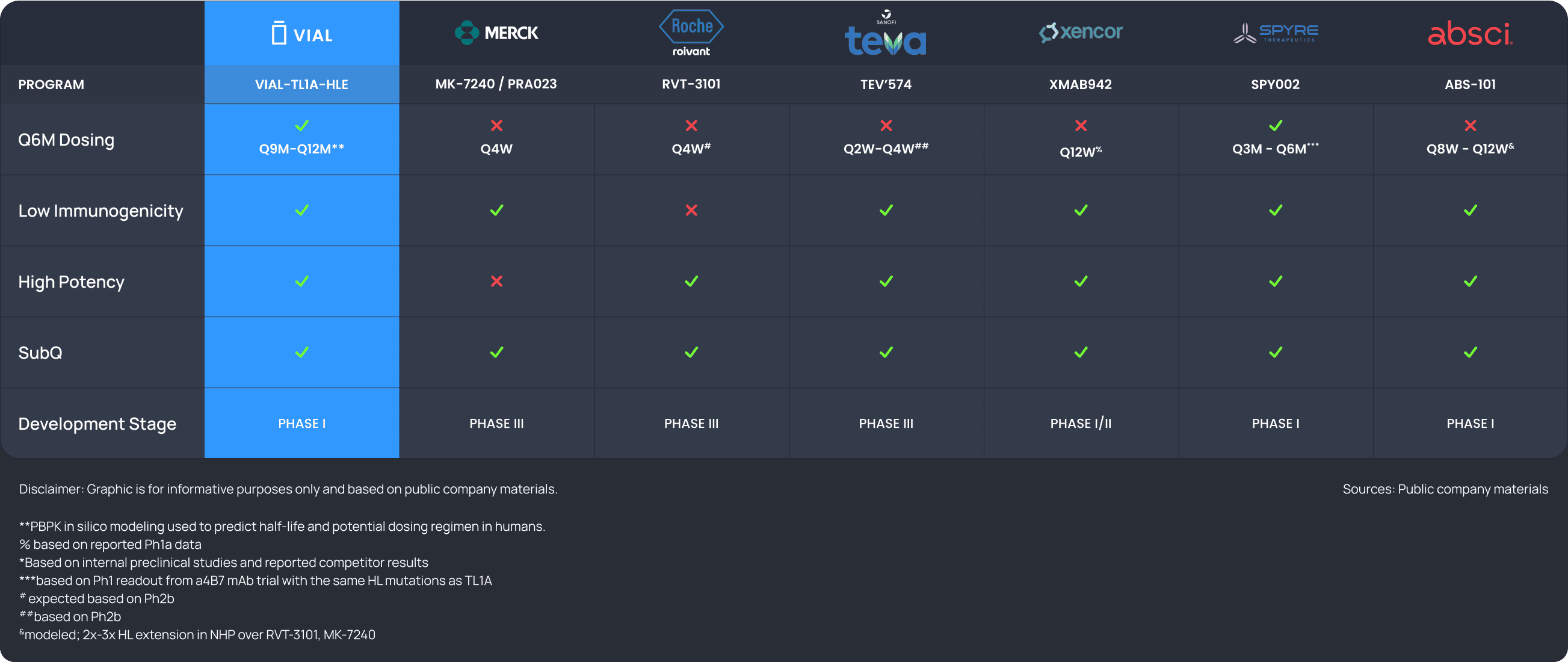
VIAL-TL1A-HLE is potentially a best-in-class anti-TL1A mAb with an extended half-life to support Q9-12M dosing, powered by Vial’s HLE platform. The program is also applicable to MASH, Atopic Dermatitis, SSc-ILD, Rheumatoid Arthritis, Hidradenitis Suppurativa, among others.
Updates
So 1st of all, TL1A Is one of the hot targets at the moment for both Crohn's and Uc. And one of the major challenges that we have in the clinic is to have drugs that need frequent administration and patients like to have drugs that are very effective and that are super safe, and that are easy to administer.
So instead of every 2 weeks, every month, having an injection every 6 months, or once a year, with a very safe mechanism of action. I mean, this is the ideal thing to have for a patient perspective and also for a doctor.
A long half-life reduces the burden on the patient hopefully provides a very steady state concentration of drug and binding of the target over the extended half-life, which in theory should be very good at preventing episodic flares.
I mean, IBD is a lifelong condition that requires long-term management. Many patients struggle with treatment, fatigue, adherence, challenges, and disruptions in daily life. So an extended half-life TL1A therapeutic, especially one with the potential for once yearly dosing, could significantly improve the quality of life, of reducing injection burden, minimizing clinic visits and maintaining consistent disease control.