Preclinical data demonstrate the potential for best-in-class anti-TL1A dosing convenience.
Interim subcutaneous safety and pharmacokinetic data from healthy volunteers anticipated in H2 2025.
Vial, a clinical-stage biotechnology company, announced that it has initiated dosing of healthy volunteers in its first clinical trial for a novel subcutaneous (SQ), extended half-life monoclonal antibody (mAb) targeting TL1A, which is being developed as a treatment for people living with moderate-to-severe IBD, and other I&I diseases.
“The initiation of the Phase 1 healthy volunteer trial for Vial’s half-life extended TL1A program is a milestone on our journey of advancing next-generation biologics programs. We look forward to engaging the IBD community with the interim data from the TL1A-VIAL-HLE Phase 1.”
Simon Burns, CEO of Vial
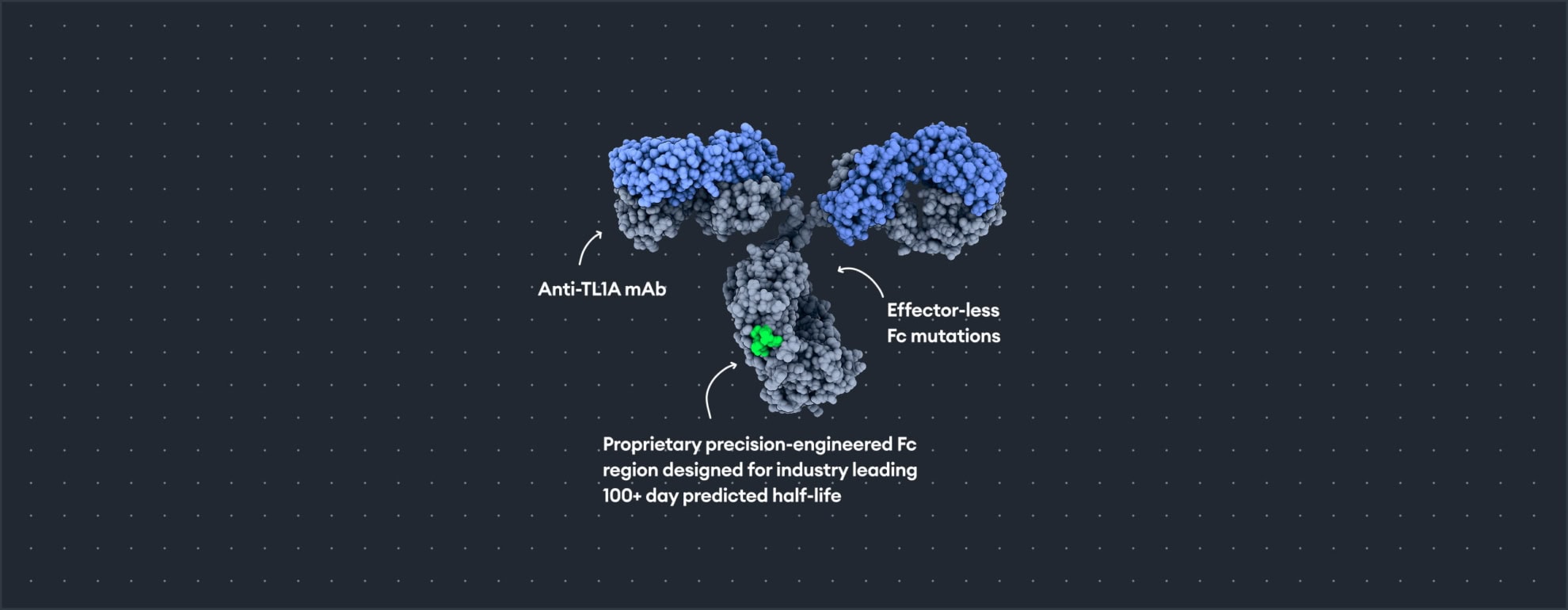
Vial’s anti-TL1A program (TL1A-VIAL-HLE) is a novel, SQ, extended half-life mAb targeting TL1A, a target with clinical validation across Ulcerative Colitis and Crohn’s Disease. TL1A-VIAL-HLE has improved binding affinity for TL1A as compared to the first and second-generation TL1A mAbs as well as demonstrated a strong inhibition of TL1A mediated apoptosis of TF-1 cells.
The TL1A-VIAL-HLE Phase 1 trial is a first-in-human, single-ascending dose trial in healthy volunteers. The study will evaluate the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of a SQ dose of TL1A-VIAL-HLE. Vial expects interim data from the trial in the second half of 2025, and, pending positive results from the Phase 1 trial and plans to initiate a multi-region Phase 2 trial in IBD later in 2025.
“TL1A inhibition has the potential to target both inflammation and fibrosis with clinical data supporting compelling efficacy in IBD. By leveraging our proprietary half-life extension technology, TL1A-VIAL-HLE has the potential to deliver much needed convenience to patients suffering from this chronic disease. We have shown in head-to-head preclinical studies with first-generation and second-generation anti-TL1A antibodies that TL1A-VIAL-HLE has the potential for best-in-class half-life with improved durability.”
Preeti Sareen, Ph.D., Director of Strategy
Preclinical data demonstrate the potential for improved dosing over other treatment options in development, including the potential for best-in-class dosing convenience compared to every other second-generation anti-TL1A in development.
About Vial
Vial is a clinical-stage biotech company based in San Francisco that has raised $100M+ to date from leading life sciences investors including General Catalyst, Buckley Ventures and Byers Capital. Vial is focused on advancing a pipeline of potentially best-in-class programs across areas of unmet need. Founded in 2020, Vial has over 60 employees across R&D, Clinical Development, Clinical Operations, Engineering, Product and Design and is actively hiring to support the scaling of core functions. More at vial.com